Select All the Reactions That Occur in a Syn Fashion.
Reactions of Alkenes |
|---|
Improver Reactions of Alkenes
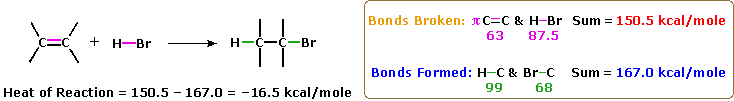
The most common chemic transformation of a carbon-carbon double bond is the add-on reaction. A large number of reagents, both inorganic and organic, accept been plant to add together to this functional group, and in this department nosotros shall review many of these reactions. A majority of these reactions are exothermic, due to the fact that the C-C pi-bail is relatively weak (ca. 63 kcal/mole) relative to the sigma-bonds formed to the atoms or groups of the reagent. Recollect, the bail energies of a molecule are the energies required to break (homolytically) all the covalent bonds in the molecule. Consequently, if the bond energies of the product molecules are greater than the bond energies of the reactants, the reaction will be exothermic. The post-obit calculations for the addition of H-Br are typical. Note that by convention exothermic reactions take a negative heat of reaction.
1. Addition of Strong Brønsted Acids
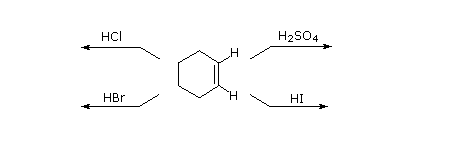
As illustrated past the preceding general equation, strong Brønsted acids such as HCl, HBr, Hullo & H2SO4, rapidly add together to the C=C functional group of alkenes to give products in which new covalent bonds are formed to hydrogen and to the conjugate base of the acid. Using the above equation as a guide, write the addition products expected on reacting each of these reagents with cyclohexene.
 |
Weak Brønsted acids such as water (pKa = xv.7) and acetic acrid (pKa = 4.75) exercise non normally add together to alkenes. However, the addition of a potent acrid serves to catalyze the improver of h2o, and in this manner alcohols may be prepared from alkenes. For example, if sulfuric acrid is dissolved in h2o it is completely ionized to the hydronium ion, H3O(+), and this strongly acidic (pKa = -1.74) species effects hydration of ethene and other alkenes.
CH2=CHii + H3O (+) —— > HCHii–CH2 OH + H (+)
The importance of choosing an appropriate solvent for these addition reactions should now exist clear. If the addition of HCl, HBr or How-do-you-do is desired, water and alcohols should not be used. These stiff acids volition ionize in such solvents to give ROH2 (+) and the nucleophilic oxygen of the solvent will compete with the halide anions in the last step, giving alcohol and ether products. By using inert solvents such every bit hexane, benzene and methylene chloride, these competing solvent additions are avoided. Because these additions proceed by way of polar or ionic intermediates, the charge per unit of reaction is greater in polar solvents, such as nitromethane and acetonitrile, than in non-polar solvents, such equally cyclohexane and carbon tetrachloride.
Regioselectivity and the Markovnikov Rule
Only one product is possible from the add-on of these strong acids to symmetrical alkenes such every bit ethene and cyclohexene. However, if the double bond carbon atoms are not structurally equivalent, every bit in molecules of 1-butene, ii-methyl-ii-butene and 1-methylcyclohexene, the reagent conceivably may add together in two dissimilar ways. This is shown for 2-methyl-ii-butene in the following equation.
| (CH3)2C=CHCH3 + H-Cl |  | (CH3)2CH–CHClCH3 | or | (CHiii)2CCl–CHHCH3 |
| ii-methyl-two-butene | 2-chloro-3-methylbutane | 2-chloro-2-methylbutane |
When addition reactions to such unsymmetrical alkenes are carried out, we observe that one of the 2 possible constitutionally isomeric products is formed preferentially. Selectivity of this sort is termed regioselectivity. In the to a higher place example, 2-chloro-two-methylbutane is nearly the sectional product. Similarly, i-butene forms 2-bromobutane as the predominant product on treatment with HBr.

After studying many addition reactions of this kind, the Russian chemist Vladimir Markovnikov noticed a trend in the structure of the favored addition product. He formulated this trend as an empirical dominion we now call The Markovnikov Dominion: When a Brønsted acrid, HX, adds to an unsymmetrically substituted double bond, the acidic hydrogen of the acid bonds to that carbon of the double bond that has the greater number of hydrogen atoms already attached to it.
In more homelier vernacular this dominion may be restated equally, "Them that has gits."
| It is a helpful practise to predict the favored production in examples such every bit those shown below: | |
|---|---|
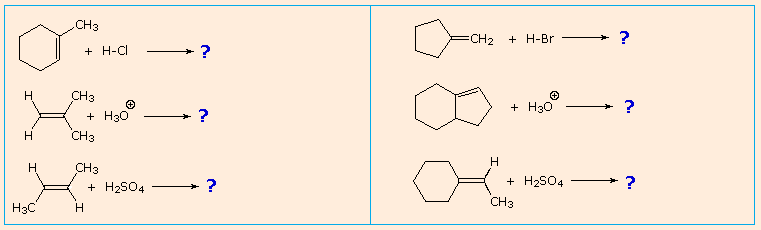 |
Empirical rules like the Markovnikov Rule are useful aids for remembering and predicting experimental results. Indeed, empirical rules are often the outset step toward practical mastery of a subject area, but they seldom constitute true understanding. The Markovnikov Rule, for case, suggests there are common and important principles at work in these add-on reactions, but it does non tell usa what they are. The side by side footstep in achieving an understanding of this reaction must be to construct a rational mechanistic model that can be tested by experiment.
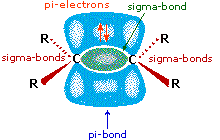 All the reagents discussed hither are strong Brønsted acids so, as a first step, it seems sensible to discover a base with which the acid tin can react. Since we know that these acids do not react with alkanes, it must be the pi-electrons of the alkene double bond that serve every bit the base. Equally shown in the diagram on the right, the pi-orbital extends into the space immediately above and below the plane of the double bond, and the electrons occupying this orbital may be attracted to the proton of a Brønsted acid. The resulting acrid-base of operations equilibrium generates a carbocation intermediate (the conjugate acrid of the alkene) which then combines rapidly with the anionic conjugate base of the Brønsted acid. This 2-step mechanism is illustrated for the reaction of ethene with hydrogen chloride by the following equations.
All the reagents discussed hither are strong Brønsted acids so, as a first step, it seems sensible to discover a base with which the acid tin can react. Since we know that these acids do not react with alkanes, it must be the pi-electrons of the alkene double bond that serve every bit the base. Equally shown in the diagram on the right, the pi-orbital extends into the space immediately above and below the plane of the double bond, and the electrons occupying this orbital may be attracted to the proton of a Brønsted acid. The resulting acrid-base of operations equilibrium generates a carbocation intermediate (the conjugate acrid of the alkene) which then combines rapidly with the anionic conjugate base of the Brønsted acid. This 2-step mechanism is illustrated for the reaction of ethene with hydrogen chloride by the following equations.
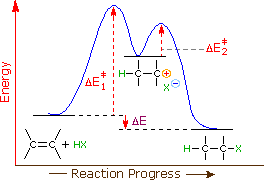 An energy diagram for this 2-step addition mechanism is shown to the left. From this diagram nosotros see that the slow or rate-determining step (the beginning footstep) is also the product determining step (the anion will necessarily bond to the carbocation site). Electron donating double bail substituents increment the reactivity of an alkene, every bit evidenced past the increased charge per unit of hydration of two-methylpropene (ii alkyl groups) compared with 1-butene (1 alkyl grouping). Evidently, alkyl substituents deed to increase the rate of add-on by lowering the activation energy, ΔE‡ ane of the charge per unit determining step, and information technology is here we should look for a rationalization of Markovnikov'south rule.
An energy diagram for this 2-step addition mechanism is shown to the left. From this diagram nosotros see that the slow or rate-determining step (the beginning footstep) is also the product determining step (the anion will necessarily bond to the carbocation site). Electron donating double bail substituents increment the reactivity of an alkene, every bit evidenced past the increased charge per unit of hydration of two-methylpropene (ii alkyl groups) compared with 1-butene (1 alkyl grouping). Evidently, alkyl substituents deed to increase the rate of add-on by lowering the activation energy, ΔE‡ ane of the charge per unit determining step, and information technology is here we should look for a rationalization of Markovnikov'south rule.
As expected, electron withdrawing substituents, such as fluorine or chlorine, reduce the reactivity of an alkene to add-on by acids (vinyl chloride is less reactive than ethene).
 | Energy |
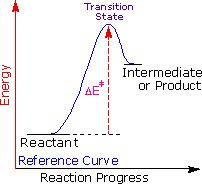
George Hammond formulated a useful principle that relates the nature of a transition country to its location on the reaction path. This Hammond Postulate states that a transition state volition be structurally and energetically similar to the species (reactant, intermediate or product) nearest to it on the reaction path. In strongly exothermic reactions the transition state will resemble the reactant species. In strongly endothermic conversions, such as that shown to the right, the transition country will resemble the high-free energy intermediate or product, and will track the energy of this intermediate if it changes. This change in transition state free energy and activation energy as the stability of the intermediate changes may be observed past clicking the higher or lower buttons to the right of the energy diagram. Iii examples may be examined, and the reference curve is changed to gray in the diagrams for higher (magenta) and lower (green) energy intermediates.
The carbocation intermediate formed in the offset step of the addition reaction now assumes a fundamental role, in that it straight influences the activation energy for this step. Independent research shows that the stability of carbocations varies with the nature of substituents, in a manner similar to that seen for alkyl radicals. The exceptional stability of allyl and benzyl cations is the upshot of charge delocalization, and the stabilizing influence of alkyl substituents, although less pronounced, has been interpreted in a similar fashion.
| Carbocation Stability | CHiii (+) | < | CHiiiCH2 (+) | < | (CHiii)iiCH(+) | ≈ | CH2=CH-CHii (+) | < | CsixHvCHtwo (+) | ≈ | (CH3)threeC(+) |
|---|
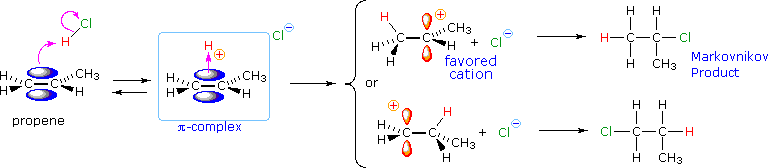
From this information, applying the Hammond Postulate, we go far at a plausible rationalization of Markovnikov'southward rule. When an unsymmetrically substituted double bond is protonated, we look the more stable carbocation intermediate to exist formed faster than the less stable culling, because the activation energy of the path to the former is the lower of the ii possibilities. This is illustrated by the following equation for the addition of hydrogen chloride to propene. Note that the initial acid-base of operations equilibrium leads to a pi-complex which immediately reorganizes to a sigma-bonded carbocation intermediate. The more stable 2º-carbocation is formed preferentially, and the conjugate base of operations of the Brønsted acid (chloride anion in the example shown below) and so rapidly bonds to this electrophilic intermediate to form the final product.
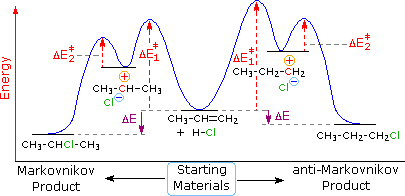
The following energy diagram summarizes these features. Annotation that the pi-complex is not shown, since this rapidly and reversibly formed species is common to both possible reaction paths.
| A more extensive discussion of the factors that influence carbocation stability may be accessed by Clicking Here. |
|---|
two. Rearrangement of Carbocations
The germination of carbocations is sometimes accompanied past a structural rearrangement. Such rearrangements accept place by a shift of a neighboring alkyl grouping or hydrogen, and are favored when the rearranged carbocation is more stable than the initial cation. The add-on of HCl to 3,3-dimethyl-i-butene, for example, leads to an unexpected production, two-chloro-ii,three-dimethylbutane, in somewhat greater yield than iii-chloro-2,2-dimethylbutane, the expected Markovnikov product. This surprising result may exist explained by a carbocation rearrangement of the initially formed 2º-carbocation to a 3º-carbocation by a i,ii-shift of a methyl group. To come across this rearrangement click the "Show Mechanism" button to the right of the equation.
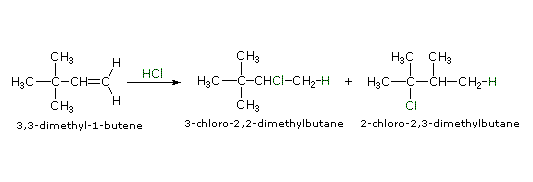 |
Some other factor that may induce rearrangement of carbocation intermediates is strain. The addition of HCl to α-pinene, the major hydrocarbon component of turpentine, gives the rearranged production, bornyl chloride, in high yield. As shown in the following equation, this rearrangement converts a 3º-carbocation to a 2º-carbocation, a transformation that is normally unfavorable. However, the rearrangement also expands a strained four-membered ring to a much less-strained v-membered ring, and this relief of strain provides a driving forcefulness for the rearrangement. A three-dimensional project view of the rearrangement may be seen by clicking the "Other View" button. The atom numbers (colored ruby) for the pinene structure are retained throughout the rearrangement to help orient the viewer. The dark-green numbers in the final product represent the proper numbering of this bicyclic ring system.
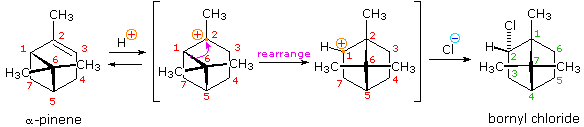 |
The propensity for structural rearrangement shown past certain molecular constitutions, as illustrated above, serves as a useful probe for the intermediacy of carbocations in a reaction. We shall employ this test later.
| An extensive and more detailed discussion of cation induced rearrangements may be accessed by Clicking Hither. |
|---|
![]()
3. Improver of Lewis Acids (Electrophilic Reagents)
The proton is non the simply electrophilic species that initiates improver reactions to the double bond. Lewis acids like the halogens, boron hydrides and certain transition metal ions are able to bond to the alkene pi-electrons, and the resulting complexes rearrange or are attacked by nucleophiles to requite improver products. The electrophilic character of the halogens is well known. Although fluorine is uncontrollably reactive, chlorine, bromine and to a bottom caste iodine react selectively with the double bond of alkenes. The addition of chlorine and bromine to alkenes, equally shown in the following general equation, proceeds by an initial electrophilic set on on the pi-electrons of the double bond. Iodine adds reversibly to double bonds, but the equilibrium does non normally favor the addition product, so it is not a useful preparative method. Dihalo-compounds in which the halogens are juxtaposed in the manner shown are called vicinal, from the Latin vicinalis, meaning neighboring.
Other halogen containing reagents which add to double bonds include hypohalous acids, HOX, and sulfenyl chlorides, RSCl. These reagents are unsymmetrical, and so their addition to unsymmetrical double bonds may in principle have place in ii means. In practice, these addition reactions are regioselective, with i of the two possible constitutionally isomeric products being favored. The electrophilic moiety of these reagents is the halogen.
(CH3)2C=CH2 + HOBr —— > (CH3)twoCOH-CHtwo Br
(CH3)twoC=CH2 + C6Hv SCl —— > (CHiii)2CCl-CH2 SC6H5
The regioselectivity of the higher up reactions may be explained by the same machinery we used to rationalize the Markovnikov dominion. Thus, bonding of an electrophilic species to the double bail of an alkene should result in preferential formation of the more stable (more highly substituted) carbocation, and this intermediate should then combine rapidly with a nucleophilic species to produce the add-on product. This is illustrated by the following equation.
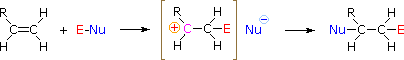
To apply this mechanism we need to determine the electrophilic moiety in each of the reagents. 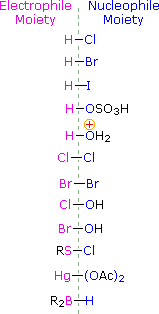 By using electronegativity differences we can dissect common add-on reagents into electrophilic and nucleophilic moieties, as shown on the right. In the case of hypochlorous and hypobromous acids (HOX), these weak Brønsted acids (pKa's ca. 8) do not react as proton donors; and since oxygen is more electronegative than chlorine or bromine, the electrophile volition be a halide cation. The nucleophilic species that bonds to the intermediate carbocation is so hydroxide ion, or more likely h2o (the usual solvent for these reagents), and the products are called halohydrins. Sulfenyl chlorides add in the opposite manner because the electrophile is a sulfur cation, RS(+), whereas the nucleophilic moiety is chloride anion (chlorine is more electronegative than sulfur).
By using electronegativity differences we can dissect common add-on reagents into electrophilic and nucleophilic moieties, as shown on the right. In the case of hypochlorous and hypobromous acids (HOX), these weak Brønsted acids (pKa's ca. 8) do not react as proton donors; and since oxygen is more electronegative than chlorine or bromine, the electrophile volition be a halide cation. The nucleophilic species that bonds to the intermediate carbocation is so hydroxide ion, or more likely h2o (the usual solvent for these reagents), and the products are called halohydrins. Sulfenyl chlorides add in the opposite manner because the electrophile is a sulfur cation, RS(+), whereas the nucleophilic moiety is chloride anion (chlorine is more electronegative than sulfur).
| If you empathise this machinery you should be able to write products for the following reactions: | |
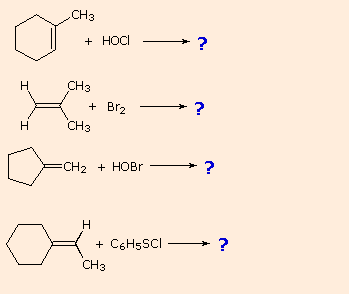 | |
The addition products formed in reactions of alkenes with mercuric acetate and boron hydrides (compounds shown at the bottom of of the reagent list) are unremarkably not isolated, but instead are converted to alcohols by a substitution reaction. These important synthetic transformations are illustrated for 2-methylpropene by the following equations, in which the electrophilic moiety is colored red and the nucleophile blue. The acme reaction sequence illustrates the oxymercuration procedure and the lesser is an example of hydroboration.
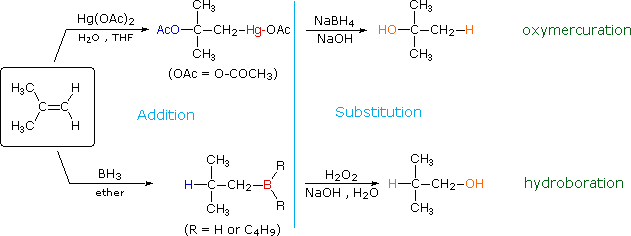
The light blue vertical line separates the addition reaction on the left from the exchange on the right. The atoms or groups that take been added to the original double bail are colored orange in the final product. In both cases the overall reaction is the addition of water to the double bond, just the regioselectivity is reversed. The oxymercuration reaction gives the product predicted by Markovnikov's rule; hydroboration on the other hand gives the "anti-Markovnikov" production. Complementary reactions such equally these are important because they allow us to direct a molecular transformation whichever way is desired.
Mercury and boron are removed from the organic substrate in the second footstep of oxymercuration and hydroboration respectively. These reactions are seldom discussed in detail; however, it is worth noting that the mercury moiety is reduced to metal mercury by borohydride (probably by way of radical intermediates), and boron is oxidized to borate by the alkaline peroxide. Addition of hydroperoxide anion to the electrophilic borane generates a tetra-coordinate boron peroxide, having the general formula R3B-O-OH(-). This undergoes successive intramolecular shifts of alkyl groups from boron to oxygen, accompanied in each event by additional peroxide addition to electron deficient boron. The retentiveness of configuration of the migrating alkyl group is attributed to the intramolecular nature of the rearrangement.
Since the oxymercuration sequence gives the same hydration product as acid-catalyzed addition of water (see Brønsted acid improver), we might question why this two-step procedure is used at all. The reason lies in the milder reaction atmospheric condition used for oxymercuration. The strong acid used for direct hydration may non be tolerated past other functional groups, and in some cases may crusade molecular rearrangement (see higher up).
The addition of borane, BHiii, requires boosted comment. In pure form this reagent is a dimeric gas BiiH6, called diborane, but in ether or THF solution it is dissociated into a solvent coordinated monomer, R2O-BHiii. Although diborane itself does non react easily with alkene double bonds, H.C. Dark-brown (Purdue, Nobel Prize 1979) discovered that the solvated monomer adds chop-chop under mild atmospheric condition. Boron and hydrogen have rather like electronegativities, with hydrogen being slightly greater, and so information technology is not likely there is significant dipolar character to the B-H bail. Since boron is electron deficient (information technology does not have a valence shell electron octet) the reagent itself is a Lewis acid and can bond to the pi-electrons of a double bail by deportation of the ether moiety from the solvated monomer. As shown in the following equation, this bonding might generate a dipolar intermediate consisting of a negatively-charged boron and a carbocation. Such a species would non exist stable and would rearrange to a neutral production past the shift of a hydride to the carbocation center. Indeed, this hydride shift is believed to occur concurrently with the initial bonding to boron, as shown by the transition state drawn below the equation, so the discrete intermediate shown in the equation is not actually formed. Nevertheless, the carbocation stability dominion cited to a higher place remains a useful fashion to predict the products from hydroboration reactions. You may correct the top equation by clicking the button on its right. Note that this addition is unique among those we have discussed, in that it is a unmarried-footstep procedure. Besides, all 3 hydrogens in borane are potentially reactive, so that the alkyl borane product from the first improver may serve equally the hydroboration reagent for two additional alkene molecules.
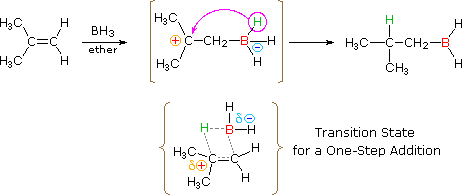 | |
To examine models of BiiH6. and its dissociation in THF
![]()
Stereoselectivity in Addition Reactions to Double Bonds
As illustrated in the cartoon on the right, the pi-bond fixes the carbon-carbon double bond in a planar configuration, and does not allow free rotation about the double bail itself.  We see then that addition reactions to this function might occur in iii dissimilar ways, depending on the relative orientation of the atoms or groups that add together to the carbons of the double bond: (i) they may bail from the aforementioned side, (ii) they may bail from opposite sides, or (3) they may bond randomly from both sides. The first two possibilities are examples of stereoselectivity, the starting time being termed syn-addition, and the second anti-add-on. Since initial electrophilic attack on the double bail may occur equally well from either side, information technology is in the 2nd step (or stage) of the reaction (bonding of the nucleophile) that stereoselectivity may be imposed.
We see then that addition reactions to this function might occur in iii dissimilar ways, depending on the relative orientation of the atoms or groups that add together to the carbons of the double bond: (i) they may bail from the aforementioned side, (ii) they may bail from opposite sides, or (3) they may bond randomly from both sides. The first two possibilities are examples of stereoselectivity, the starting time being termed syn-addition, and the second anti-add-on. Since initial electrophilic attack on the double bail may occur equally well from either side, information technology is in the 2nd step (or stage) of the reaction (bonding of the nucleophile) that stereoselectivity may be imposed.
If the 2-pace mechanism described above is correct, and if the carbocation intermediate is sufficiently long-lived to freely-rotate well-nigh the sigma-bond component of the original double bond, we would expect to detect random or non-stereoselective addition in the products. On the other hand, if the intermediate is short-lived and factors such equally steric hindrance or neighboring grouping interactions favor one side in the second step, then stereoselectivity in production germination is likely. The following table summarizes the results obtained from many studies, the formula HX refers to all the strong Brønsted acids. The interesting differences in stereoselectivity noted here provide farther insight into the mechanisms of these add-on reactions.
| Reagent | H–X | Tenii | HO–Ten | RS–Cl | Hg(OAc)2 | BH3 |
|---|---|---|---|---|---|---|
| Stereoselectivity | mixed | anti | anti | anti | anti | syn |
1. Brønsted Acid Additions
The stereoselectivity of Brønsted acid addition is sensitive to experimental conditions such every bit temperature and reagent concentration. The selectivity is frequently anti, but reports of syn selectivity and non-selectivity are not uncommon. Of all the reagents discussed here, these potent acrid additions (E = H in the post-obit equation) come up closest to proceeding by the proposed two-step mechanism in which a discrete carbocation intermediate is generated in the first step. Such reactions are nearly prone to rearrangement when this is favored by the alkene structure.

2. Addition Reactions Initiated by Electrophilic Halogen
The halogens chlorine and bromine add rapidly to a wide variety of alkenes without inducing the kinds of structural rearrangements noted for strong acids (starting time example below). The stereoselectivity of these additions is strongly anti, as shown in many of the post-obit examples.
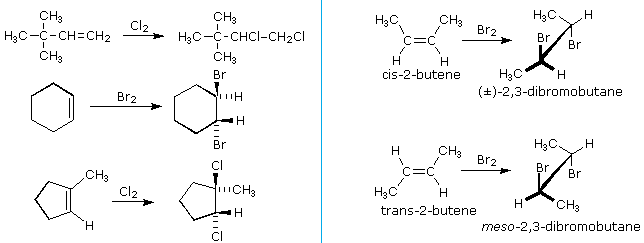
An important principle should be restated at this fourth dimension. The alkenes shown here are all achiral, merely the addition products have chiral centers, and in many cases may exist as enantiomeric stereoisomers. In the absenteeism of chiral catalysts or reagents, reactions of this kind will always requite racemic mixtures if the products are enantiomeric. On the other hand, if two chiral centers are formed in the addition the reaction volition be diastereomer selective. This is conspicuously shown past the addition of bromine to the isomeric two-butenes. Anti-add-on to cis-2-butene gives the racemic product, whereas anti-addition to the trans-isomer gives the meso-diastereomer.
We can account both for the loftier stereoselectivity and the lack of rearrangement in these reactions past proposing a stabilizing interaction between the developing carbocation center and the electron rich element of group vii atom on the adjacent carbon. This interaction, which is depicted for bromine in the post-obit equation, delocalizes the positive charge on the intermediate and blocks halide ion assault from the syn-location.
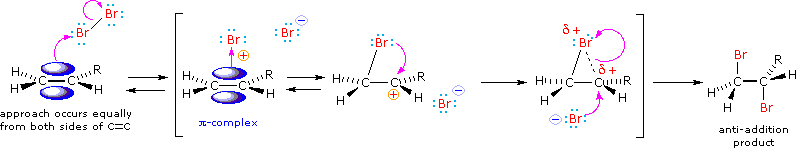
The stabilization provided by this halogen-carbocation bonding makes rearrangement unlikely, and in a few cases three-membered cyclic halonium cations have been isolated and identified every bit true intermediates. A resonance description of such a bromonium ion intermediate is shown beneath. The positive charge is delocalized over all the atoms of the band, but should be concentrated at the more substituted carbon (carbocation stability), and this is the site to which the nucleophile will bond.
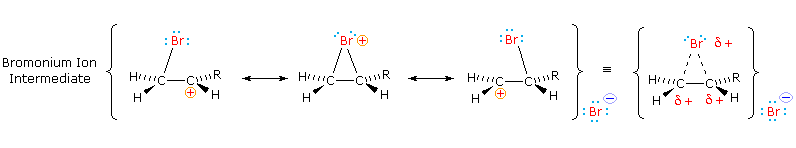
| The stereoselectivity described here is in large function due to a stereoelectronic result. |
|---|
Considering they go along by manner of polar ion-pair intermediates, chlorine and bromine addition reactions are faster in polar solvents than in non-polar solvents, such as hexane or carbon tetrachloride. Withal, in order to preclude solvent nucleophiles from competing with the halide anion, these non-polar solvents are often selected for these reactions. In water or alcohol solution the nucleophilic solvent may open the bromonium ion intermediate to give an α-halo-booze or ether, together with the expected vic-dihalide. Such reactions are sensitive to pH and other factors, and so when these products are desired information technology is necessary to modify the addition reagent. Aqueous chlorine exists equally the following equilibrium, Yardeq ≈ ten-four. Past adding AgOH, the concentration of HOCl can exist profoundly increased, and the chlorohydrin add-on product obtained from alkenes.
| Cl2 + H2O |  | HOCl + HCl |
The more than widely used HOBr reagent, hypobromous acid, is commonly fabricated by hydrolysis of Due north-bromoacetamide, as shown beneath. Both HOCl and HOBr additions occur in an anti fashion, and with the regioselectivity predicted by this machinery (OH bonds to the more substituted carbon of the alkene).
| CHthreeCONHBr + H2O |  | HOBr + CHiiiCONHtwo |
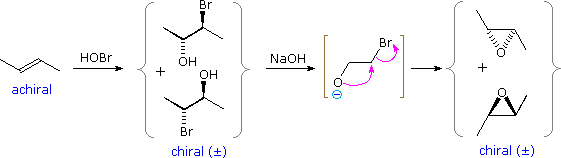
Vicinal halohydrins provide an alternative route for the epoxidation of alkenes over that of reaction with peracids. Every bit illustrated in the following diagram, a base induced intramolecular substitution reaction forms a iii-membered cyclic ether called an epoxide. Both the halohydrin germination and halide deportation reactions are stereospecific, and then stereoisomerism in the alkene will be reflected in the epoxide product (i.due east. trans-2-butene forms a trans-disubstituted epoxide). A full general procedure for forming these useful compounds will be discussed in the side by side section.
| Other Addition Reactions |
|---|
This page is the holding of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to assist in capacity edifice in chemical pedagogy. 05/05/2013
0 Response to "Select All the Reactions That Occur in a Syn Fashion."
Post a Comment